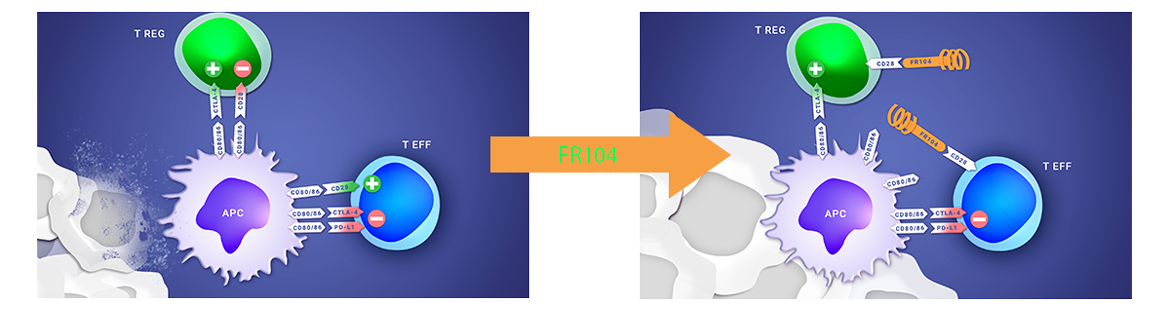
A PROPOS DU PROGRAMME CLINIQUE FR104/VEL-101
Les résultats positifs de la phase 1 clinique de FR104 ont montré des premiers signes d’efficacité, un bon profil de tolérance et ont permis de déterminer la dose optimale pour la phase 2, éléments qui soutiennent la poursuite du développement clinique du produit dans les maladies auto-immunes ou la transplantation.
Poirier et al. ; First-in-Human Study in Healthy Subjects with FR104, a Pegylated Monoclonal Antibody Fragment Antagonist of CD28 ; Journal of Immunology; 2016
FR104/VEL-101 fait l’objet d’un accord de licence mondial (avril 2021) avec Veloxis Pharmaceuticals Inc., un leader mondial de la transplantation, pour développer, fabriquer, enregistrer et commercialiser FR104/VEL-101 dans toutes les indications de transplantation. Sur la base des résultats précliniques et cliniques de phase 1 positifs, Veloxis prévoit de poursuivre le développement de FR104 pour proposer une nouvelle option thérapeutique dans la prophylaxie du rejet d’organe chez les patients ayant reçu une transplantation d’organe solide. Une étude clinique internationale de phase 2 dans la transplantation rénale est en cours de préparation par Veloxis.
Un essai de Phase 1/2 (FIRsT) est en cours, évaluant FR104, administré pour la première fois chez des patients ayant reçu une transplantation rénale. Cette étude est menée dans le cadre d’un accord de collaboration entre OSE Immunotherapeutics et le Centre Hospitalier Universitaire de Nantes, promoteur de l’essai. Le recrutement de patients dans cette étude clinique a été finalisé. Une évaluation sur le suivi à plus long terme est prévue pendant un an après la transplantation.
L’étude FIRsT vise à évaluer la sécurité, la tolérance, la pharmacocinétique, la pharmacodynamique et l’efficacité de FR104 chez des patients ayant reçu une transplantation rénale (ClinicalTrials.gov : NCT04837092).