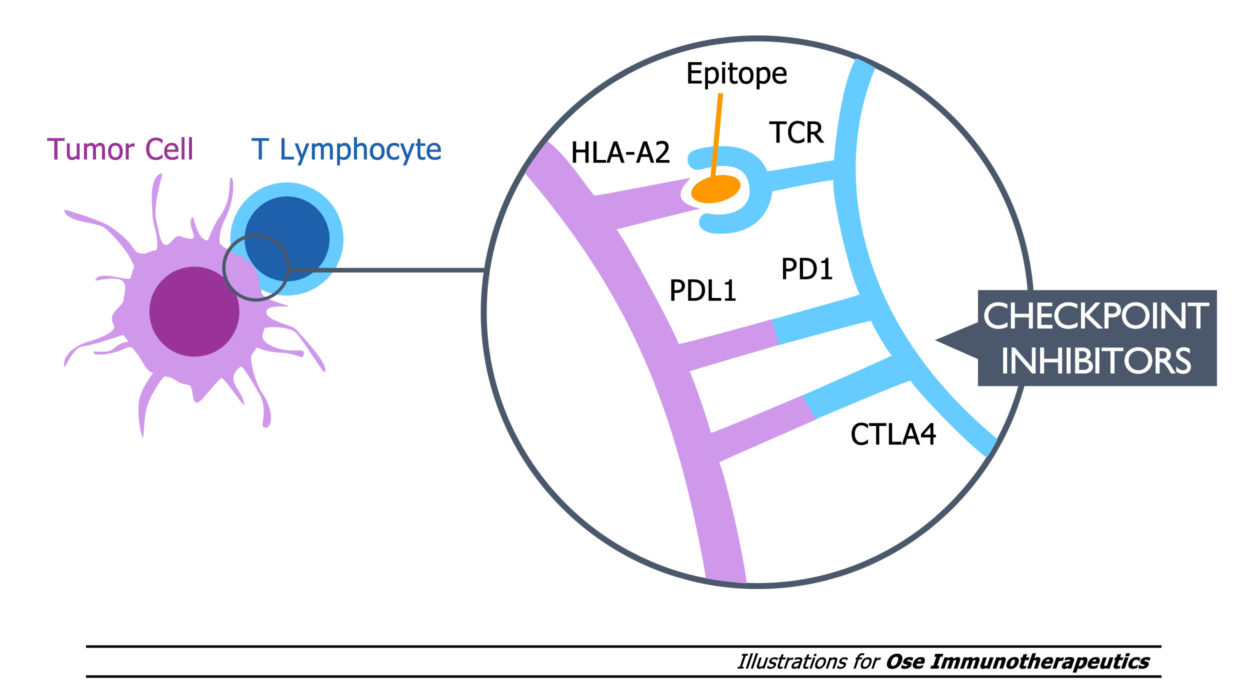
Tedopi® est un traitement qui s’adresse aux patients HLA–A2+, un récepteur clé de la réponse cytotoxique.
Tedopi® a été évalué en Phase 3 clinique chez des patients atteints d’un cancer du poumon non à petites cellules (CPNPC) après échec d’un inhibiteur de point de contrôle (IPC) – Essai nommé Atalante 1
Des résultats cliniques de Phase 3 convaincants
L’article, intitulé « Randomized Open-Label Controlled Study of Cancer Vaccine OSE2101 Versus Chemotherapy in HLA-A2-positive Patients with Advanced Non-Small Cell Lung Cancer with Resistance to Immunotherapy : ATALANTE-1 » publié dans la revue par un Comité de lecture, dans ‘Annals of Oncology’ présente les données positives de l’étude clinique internationale de Phase 3 randomisée qui montre que Tedopi®, vaccin innovant contre le cancer, améliore la survie globale avec une meilleure tolérance et une meilleure qualité de vie en monothérapie versus une chimiothérapie, chez des patients HLA-A2 positifs atteints d’un CPNPC avancé ou métastatique ayant progressé après au moins 12 semaines après un traitement séquentiel par chimiothérapie et IPC.
Principaux résultats de la première étude clinique de phase 3 de Tedopi® chez des patients HLA-A2 positifs atteint d’un cancer du poumon non à petites cellules (CPNPC)
Cet essai de phase 3 a permis de démontrer un bénéfice thérapeutique significatif chez les patients en résistance secondaire (1) aux inhibiteurs de points de contrôle (IPC), définie par des patients en échec à une chimiothérapie à base de platine suivie par un minimum de 12 semaines d’un traitement par IPC (analyse principale de l’essai). Tedopi® a montré un ratio bénéfice/risque favorable par rapport au traitement standard (docetaxel ou pemetrexed) chez les patients HLA-A2 positifs, atteints d’un CPNPC en résistance secondaire aux IPC.
Les principaux résultats ont montré :
Une meilleure efficacité
- La survie globale (critère principal) a été améliorée de façon statistiquement significative :
HR = 0.59 (95% CI : 0.38, 0.91) en faveur du bras Tedopi®, avec une réduction de 41 % du risque de décès.Un taux de survie globale à un an de 44,4 % avec Tedopi® versus 27,5 % avec la chimiothérapie. Un gain cliniquement pertinent en médiane de survie globale de 3,6 mois avec Tedopi®, soit une survie globale médiane de 11,1 mois avec Tedopi® versus 7,5 mois avec le traitement standard (p = 0,017).
- La survie après progression était aussi significativement plus longue dans le bras Tedopi® (7,7 mois versus 4,6 mois ; p = 0,004).
Un meilleur profil de tolérance et une meilleure qualité de vie
- Le score de performance ECOG PS (2), de l’état général maintenu avec un temps jusqu’à détérioration significativement plus long dans le bras Tedopi® (9.0 versus 3.3 mois ; p=0.006 ; HR = 0.43).
- Une meilleure qualité de vie observée avec Tedopi® (p= 0,04) (État général : p = 0,045 ; Principales fonctions : p = 0,025).
- Un bon profil de tolérance de Tedopi® avec moins d’effets indésirables de grade 3-5 (Tedopi® : 38 % versus traitement standard : 68 % ; p < 0,001).
(1) La résistance secondaire est définie par un échec après un minimum de 12 semaines de traitement par IPC en séquentiel avec une chimiothérapie (Kluger H et al ; Journal for immunoTherapy of Cancer 2020 définissant la résistance tumorale au blocage de la voie PD1 : recommandations de la première Taskforce du SITC sur la résistance à l’immunothérapie).
(2) Le score ECOG est une échelle de performance permettant d’évaluer l’état de santé général d’un patient. Elle est sous-divisée en 5 grades allant pleinement actif (0), à totalement invalide puis au décès (5).
Ces résultats cliniques positifs issus d’une première étude de Phase 3 dans une population cible clairement définie sont basés sur un fort rationnel biologique : la réponse accrue des cellules T spécifiques induite par le mécanisme d’activation innovant de Tedopi® est corrélée à une amélioration de la survie globale chez les patients HLA-A2+ souffrant d’un CPNPC. Cette activation T spécifique diffère du mécanisme d’action des inhibiteurs de point de contrôle qui lèvent les freins sur la réponse immune.