Tedopi®
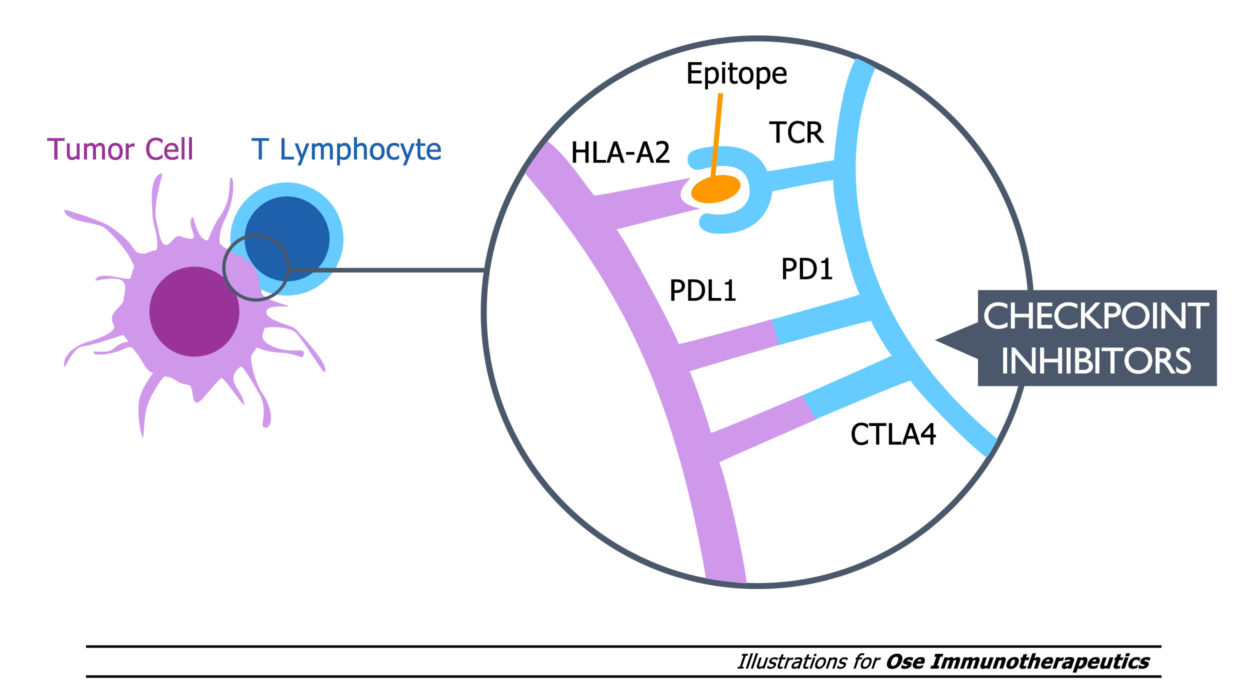
Tedopi® is a novel T-cell epitope-based cancer vaccine targeting five tumor-associated antigens, an activating and differentiated off-the-shelf immunotherapy expanding tumor specific T-lymphocytes in HLA-A2 cancer patients.
Tedopi® is evaluated in a confirmatory Phase 3 clinical trial (named Artemia) in second-line treatment of non-small cell lung cancer (NSCLC) in HLA-A2 patients with secondary resistance to immune checkpoint inhibitors (ICI).
This pivotal study aims at supporting the registration of Tedopi® in second-line treatment of NSCLC in Europe and North America, in parallel with the companion diagnostic test for HLA-A2 positive patients.
This international study is being conducted in the United States, Canada, Europe and United Kingdom and is planned to include 363 patients.
Tedopi® is also being investigated in other Phase 2 trials:
ABOUT THE TEDOPI® CLINICAL PROGRAM
Tedopi® is being evaluated in three major cancer indications:
Artemia is an international, randomized, open-label Phase 3 trial comparing the efficacy and safety of Tedopi® monotherapy versus standard of care in HLA-A2 positive patients with metastatic NSCLC with secondary resistance (1) to ICI. The primary endpoint is overall survival. This confirmatory pivotal trial will include 363 patients, and aims at supporting the regulatory registration of Tedopi® in second-line treatment of NSCLC in the United States, Canada, Europe and United Kingdom.
(1) After at least 12 weeks of ICI maintenance treatment without cytotoxic therapy (Task force SITC 2020 – Kluger H et al. 2020). Kluger et al. 2023
Clinicaltrials.gov: NCT06472245
The Artemia trial, the last registration development step of Tedopi®, is supported by the positive and promising results from the first Phase 3, named Atalante-1, in third-line treatment of NSCLC.
This randomized, international Phase 3 clinical trial had demonstrated a significant therapeutic benefit in patients with secondary resistance to immune checkpoint inhibitors (ICI) defined as patients with failure to platinum-based chemotherapy followed by a minimum of 12 weeks ICI treatment (main analysis of the trial). Tedopi® demonstrated a favorable benefit/risk ratio versus standard of care (SoC) docetaxel or pemetrexed in advanced HLA-A2+ NSCLC patients with secondary resistance to ICI.
An article, titled “Randomized Open-Label Controlled Study of Cancer Vaccine OSE2101 Versus Chemotherapy in HLA-A2-positive Patients with Advanced Non-Small Cell Lung Cancer with Resistance to Immunotherapy: ATALANTE-1” published in the peer-reviewed publication Annals of Oncology featured these positive results.
Clinicaltrials.gov: NCT02654587
This three-arm Phase 2 study evaluates neo-epitope-based vaccine Tedopi® in combination with Bristol Myers Squibb’s Opdivo® (nivolumab), an immune checkpoint inhibitor, or Tedopi® in combination with chemotherapy versus chemotherapy alone as second-line treatment in HLA-A2 positive patients with metastatic NSCLC after first-line chemo-immunotherapy. The primary endpoint of the study is the 1-year survival rate.
Clinicaltrials.gov: NCT04884282
The Phase 2 study TEDOPaM aims at comparing Tedopi® in combination with FOLFIRI chemotherapy versus FOLFIRI, in maintenance treatment after treatment with FOLFIRINOX. The primary endpoint of the trial is the one-year survival rate.
Clinicaltrials.gov : NCT03806309
The three-arm TEDOVA Phase 2 study evaluates Tedopi® as a maintenance treatment, alone or in combination with the anti-PD-1 Keytruda®, versus the best supportive care in platinum-sensitive recurrent ovarian cancer patients, with controlled disease after platinum-based chemotherapy. The primary endpoint of the study is the progression free survival rate.
Clinicaltrials.gov: NCT04713514