Tedopi®
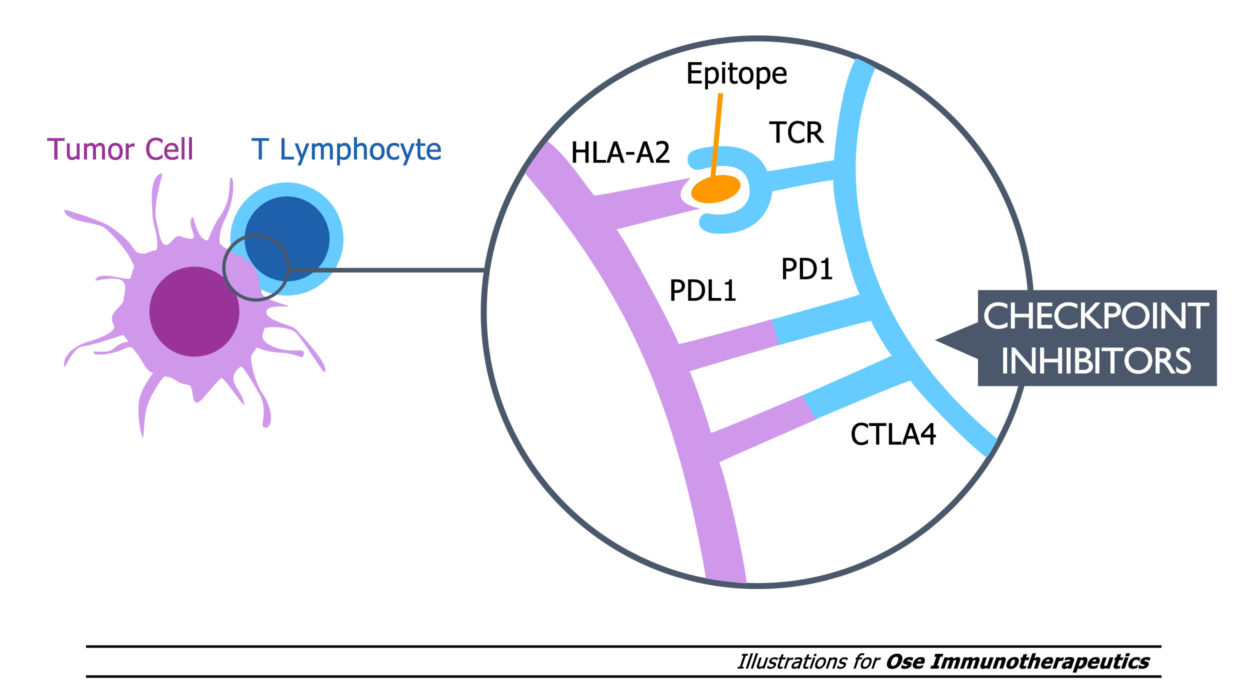
Tedopi® is a novel T-cell epitope-based cancer vaccine targeting five tumor-associated antigens, an activating and differentiated off-the-shelf immunotherapy expanding tumor specific T-lymphocytes in HLA-A2 cancer patients.
Tedopi® has been evaluated in a clinical Phase 3 study in patients with non-small cell lung cancer (NSCLC) after immune checkpoint inhibitor failure (ICI) – Trial named Atalante-1.
Compelling Phase 3 Clinical Data
Tedopi® is the first cancer vaccine to show clinically meaningful efficacy results associated with a better safety and quality of life profile in monotherapy versus active comparator (chemotherapy-based standard of care) post-ICI failure in advanced or metastatic NSCLC.
An article, titled “Randomized Open-Label Controlled Study of Cancer Vaccine OSE2101 Versus Chemotherapy in HLA-A2-positive Patients with Advanced Non-Small Cell Lung Cancer with Resistance to Immunotherapy: ATALANTE-1” published in the peer-reviewed publication in Annals of Oncology features positive data from the randomized international Phase 3 study showing that novel cancer vaccine Tedopi® improves overall survival with a better safety and quality of life profile in monotherapy compared to chemotherapy in HLA-A2 positive patients with advanced or metastatic NSCLC who have progressed at least 12 weeks after sequential treatment with chemotherapy and ICI.
Main results of the first Phase 3 clinical trial of Tedopi® in HLA-A2+ patients with NSCLC
This Phase 3 clinical trial has demonstrated a significant therapeutic benefit in patients with secondary resistance (1) to immune checkpoint inhibitors (ICI) defined as patients with failure to platinum-based chemotherapy followed by a minimum of 12 weeks ICI treatment (main analysis of the trial). Tedopi® demonstrated a favorable benefit/risk ratio versus standard of care (SoC) docetaxel or pemetrexed in advanced HLA-A2+ NSCLC patients with secondary resistance to ICI.
The main results were:
Improved efficacy
Improved safety profile and Quality of Life
(1) Secondary resistance is defined as failure after a minimum of 12 weeks of Immune checkpoint inhibitor given in sequential chemotherapy – checkpoint inhibitors treatment (Kluger HM et al; Journal for immunoTherapy of Cancer 2020 Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce)
(2) The ECOG score is a performance scale used to quantify the general health condition of a patient. It is subdivided into 5 grades from 0 to 5, ranging from fully active (0) to fully disabled, then to death (5).
These positive clinical results in a clearly-defined target population for this first Phase 3 trial are based on a strong biological rationale: increased specific T-cell responses induced by Tedopi®’s innovative mechanism of action correlated to the overall survival in HLA-A2+ NSCLC patients. The direct activation of tumor specific T-cells by Tedopi® differs from ICI releasing the break of immune response.
Tedopi® is also being investigated in other Phase 2 trials:
ABOUT THE TEDOPI® CLINICAL PROGRAM
Tedopi® is being evaluated in three major cancer indications:
The Atalante-1 clinical trial of Tedopi® evaluated the benefit of the product in an HLA-A2 positive patient population with NSCLC at invasive stage IIIB or metastatic stage IV, in 2nd or 3rd line treatment following checkpoint inhibitor failure. The Tedopi® treatment was compared to docetaxel or pemetrexed chemotherapy (CT) treatments in this patient population, with overall survival as the primary endpoint of the trial.
Clinicaltrials.gov: NCT02654587
A confirmatory Phase 3 study with a companion diagnostic strategy is under preparation to support the registration of Tedopi® as a potential new standard of care in second line for non-small cell lung cancer (NSCLC) patients in secondary resistance to immune checkpoint inhibitors (ICI) based on positive regulatory advice from FDA and EMA.
Phase 2 in NSCLC in combination with Opdivo® (nivolumab) (sponsor FoRT)
This three-arm Phase 2 study evaluates neo-epitope-based vaccine Tedopi® in combination with Bristol Myers Squibb’s Opdivo® (nivolumab), an immune checkpoint inhibitor, or Tedopi® in combination with chemotherapy versus chemotherapy alone as second-line treatment in HLA-A2 positive patients with metastatic NSCLC after first-line chemo-immunotherapy. The primary endpoint of the study is the 1-year survival rate.
Clinicaltrials.gov: NCT04884282
The Phase 2 study TEDOPaM aims at comparing Tedopi® in combination with FOLFIRI chemotherapy versus FOLFIRI, in maintenance treatment after treatment with FOLFIRINOX. The primary endpoint of the trial is the one-year survival rate.
Clinicaltrials.gov : NCT03806309
The three-arm TEDOVA Phase 2 study evaluates Tedopi® as a maintenance treatment, alone or in combination with the anti-PD-1 Keytruda®, versus the best supportive care in platinum-sensitive recurrent ovarian cancer patients, with controlled disease after platinum-based chemotherapy. The primary endpoint of the study is the progression free survival rate.
Clinicaltraisl.gov: NCT04713514