Lusvertikimab (OSE-127)
a humanized monoclonal antibody, is an antagonist of the alpha chain of the interleukine-7 receptor, CD127, present on T effector cells, thus down regulating the immune activity.
Lusvertikimab (OSE-127) is a monoclonal immuno-modulatory antibody targeting the CD127 receptor, the alpha chain of the interleukin-7 receptor (IL-7R) that induces a powerful antagonist effect on effector T lymphocytes. Interleukin-7 is a cytokine which specifically regulates the tissue migration of human effector T lymphocytes. The blockage of IL-7R prevents the migration of pathogenic T lymphocytes while preserving regulator T lymphocytes which have a positive impact in autoimmune diseases.
Lusvertikimab is under a Phase 2 clinical trial, named CoTikiS, in patients with moderate to active Ulcerative Colitis. Full Phase 2 efficacy and safety data from the induction period of CoTikiS were presented at the 20th Congress of ECCO (European Crohn’s and Colitis Organization) in Berlin in February 2025, demonstrating meaningfull efficacy and a favorable safety profile.
Besides immuno-inflammation, Lusvertikimab has also demonstrated great therapeutic potential in immuno-oncology through positive efficacy preclinical results in Acute Lymphoblastic Leukemia (ALL), a very aggressive tumor. Novel targeted immunotherapies are urgently needed to address relapsed/refractory (R/R) form of the disease, especially in T-ALL where the need for novel therapies is significant.
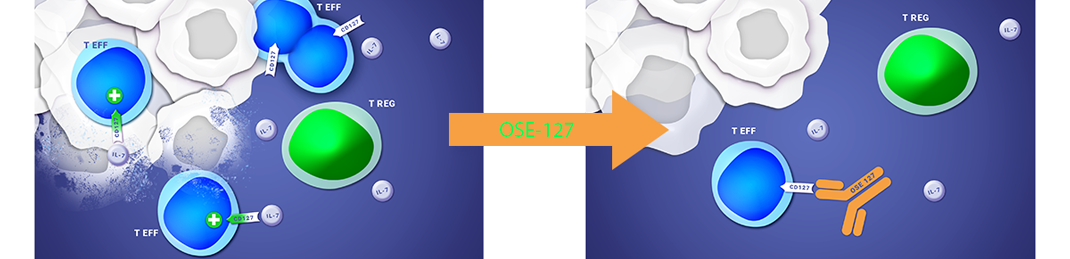
ABOUT THE LUSVERTIKIMAB (OSE-127) CLINICAL PROGRAM
The randomized, double-blind Phase 2 clinical trial CoTikiS has evaluated the efficacy and the safety of Lusvertikimab versus placebo in 136 patients with moderate to severe active UC who failed or lost response to previous treatment(s)*. CoTikiS is a 50-week study, with a 10-week induction period evaluating two doses (450mg or 850mg) of Lusvertikimab against placebo, a 24-week additional open label treatment extension period (OLE) during which all subjects received Lusvertikimab 850mg infusions every 4 weeks and a 16-week safety follow-up period free of treatment.
*Previous corticosteroids, immunosuppressive agents or previous biological treatments.
Positive results from Phase 1 clinical study
The Phase 1 had shown a good safety and tolerability profile for Lusvertikimab
An article, selected as ‘Top Read’ for the March 15th issue, was published online in ‘The Journal of Immunology’ (online). The publication, entitled
First-in-Human Study in Healthy Subjects with the Non-Cytotoxic 1 Monoclonal Antibody OSE-127, a Strict Antagonist of the IL-7Rα
reports on the Phase 1 positive results. These showed a good safety and tolerability profile for Lusvertikimab (OSE-127), with no signs of significant lymphopenia, cytokine release syndrome or T-cell compartment alterations. All pharmacokinetic and pharmacodynamic parameters were consistent and demonstrated a dose-proportionality across the several dose-levels up to 10 mg/kg. A decreased IL-7 pathway gene signature in human peripheral blood cells has been demonstrated confirming the efficient blockade of the target.
UC is a debilitating and chronic inflammatory bowel disease which affects 3.3 million patients in US, Europe and Japan (1) representing 12.2 per 100,000 people by year (2). Despite broad options, remission rates are only 25-30% (3) leaving most patients without satisfactory treatments. The disease is characterized by a heavy burden on patients’ lives with a strong medical need for new therapeutic options.
(1) EvaluatePharma
(2) Updated Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota (1970-2011). Loftus EV et al. October 2014.
(3) Drugs Context. 2019; 8: 212572 –doi: 10.7573/dic.212572